Federal Updates for Researchers
Please visit our webpage often for the latest updates about impacts on research. Last updated Jan 20th.
Learn moreChapter 5: Post Award - Administrative Requirements
Chapter 5 outlines the administrative requirements for managing sponsored projects, focusing on compliance with sponsor regulations and federal guidelines. It covers essential processes such as obtaining prior approval for changes, managing property, submitting progress reports, and ensuring proper record retention.
During the period of performance of an award, it may become necessary to deviate from the accepted budget or project plan. Many changes require sponsor prior approval before they can be initiated, while other changes may be made without sponsor approval. Requirements for prior approval vary among sponsors, and PIs and their administrative units are responsible for complying with all sponsor requirements. Non-compliance with prior approval requirements can result in disallowed costs, and sponsors may impose additional penalties, such as award termination, depending on the severity of the non-compliant action.
For federal awards, all prior approval requests must be submitted to the sponsor by OGC. PIs and administrative units are responsible for developing the prior approval request, and OGC Post Award will formally submit the request. For non-federal awards, the requirements for submitting prior approval requests will vary.
The award terms and conditions generally identify prior approval requirements or provide references to where these requirements can be found. The following summarizes where to find prior approval requirements outside of the award document:
- Federal awards – 2 CFR 200.308(f) identifies general prior approval requirements for most federal awards and 2 CFR 200.407 provides a list of administrative actions and selected items of cost that may require prior approval
- NIH – Prior approval requirements are found in Section 8.1.2 of the NIH Grants Policy Statement (NIH GPS)
- NSF – Prior approval requirements are found in Chapter VII of the NSF PAPPG
- Non-federal awards – The award term and conditions, sponsor guidelines, or sponsor website should provide prior approval requirements
5.1.1 Prior Approval Requirements Common to Most Federal Awards
2 CFR 200.308(f) identifies prior approval requirements applicable to most federal awards, which includes:
- Change in scope or the objective of the project
- Change in key personnel, including employees and contractors, that are identified by name or position in the notice of award
- The disengagement from a project for more than 3 months, or a 25 percent reduction in time and effort devoted to the award over the course of the period of performance by the PI
- The addition of certain items of cost or cost categories that were not included in the approved budget
- The transfer of funds budgeted for participant support costs to other budget categories
- The addition of a subagreement not included in the notice of award (For NIH awards, prior approval is not required to add a domestic cost-reimbursable subagreement)
- Changes to the approved cost-sharing amount
- Request for additional federal funds
- Transferring funds between construction and non-construction cost categories
- A no-cost extension, which is a request to extend the period of performance other than any one-time extension provided for by the award terms and conditions (Please note, the University refers to a “one-time extension” as a 1st no-cost extension)
Change in Scope
In general, PIs may make changes in the methodology, approach, or other aspects of a project’s objectives. However, changing the scope of a project almost always requires sponsor prior approval. A change in scope refers to a change in the direction, aims, objectives, purpose, or type of research training.
Potential indicators of a change in scope include, but are not limited to:
- Change in the specific aims approved at the time of award
- Substitution of one animal model for another
- Changes from the approved involvement of human subjects that would result in an increased risk
- Shift of the research emphasis from one disease area to another
- A clinical hold by the FDA under a study involving an investigational new drug or device (IND/IDE)
- Application of a new technology, such as changing assays from those approved to a different type of assay
- For NIH, purchasing a unit of equipment exceeding $25,000 that was not included in the award notice
- For NIH, significant rebudgeting when expenditures in a single direct cost budget category deviate (increase or decrease) from the categorical commitment level established for the budget period by 25 percent or more of the total costs awarded
Reduction in PI Effort
Federal regulations require prior approval when there is a reduction in PI effort in the following situations:
- When the PI is disengaged from the project for three months, or
- When there is a 25% reduction in time and effort by a PI
A PI who does not contribute effort on a project for three consecutive months must obtain prior approval from the federal sponsor to continue the project. PIs should discuss prolonged leaves of absence with the sponsor before taking a sabbatical, family or medical leave, a prolonged vacation, or other pre-planned leave that requires them to be absent for more than three months, to ensure their project will remain active upon their return. If a PI has a medical or family emergency requiring them to be absent for more than three months, the sponsor should be notified as soon as possible. One option is to name a temporary PI for the project during the original PI’s extended absence. Prior approval is required to name a temporary PI.
Prior approval is also required when there is a 25% reduction in the PI’s time and effort. The 25% reduction refers to the percentage change from the proposed time and effort. For example, if a PI proposed 20% time and effort on an award (2.4 Calendar Months), they must provide at least 15% of time and effort (1.8 Calendar Months) to retain the award without obtaining prior approval.
Unless otherwise notified in the notice of award, sponsors expect PIs to provide the level of effort outlined in the proposal, even when the amount funded is less than requested or the initial budget period is shortened.
5.1.2 Prior Approval Requirements for Non-Federal Awards
The prior approval requirements for non-federal awards vary. It is the responsibility of the PI and their administrative units to determine and comply with all sponsor requirements.
Federal research awards typically provide Expanded Authorities, which are flexibilities designed to reduce administrative burden. Under Expanded Authorities, federal agencies may waive certain prior approval requirements for research awards. Common Expanded Authorities for federal research awards include:
- Pre-Award Spending: Allows costs to be incurred, at the recipient’s risk, up to 90 days before the period of performance.
- Carry Forward / Carryover: Allows funding from one budget period to be used in the subsequent budget period.
- First No-Cost Extension: Allows the period of performance to be extended for up to 12 months. (Note: 2 CFR 200 and some federal sponsors identify a first no-cost extension as a “one-time” extension. All federal sponsors require prior approval for a second extension to an award.)
Information about University procedures for each expanded authority can be found through the hyperlinks provided in the previous list.
It is important to note that Expanded Authorities are only provided to federal research grants, not to federal contracts. Additionally, not all federal agencies provide Expanded Authorities for their awards, and prior approval may be required for any or all of the actions identified above.
Since January 2025, some federal agencies have rescinded certain expanded authorities. It is the responsibility of the PI and their administrative units to follow all current requirements for their awards.
National policy requirements, sometimes referred to as public policy requirements, are broad categories of federal laws and regulations applicable to federal awards. Examples of national policy requirements include:
- Animal welfare and protection
- Human subject protection
- Environmental safety and conservation
- Civil rights and non-discrimination
- Employee health and safety
- National security
- Domestic economic protection
- Labor standards
Applicable national policy requirements depend on the nature of the award, monetary thresholds, and the type of recipient. PIs and their administrative units are responsible for complying with all applicable national policy requirements.
Public Health Service (PHS) agencies, including NIH, are subject to annual legislative mandates included in the appropriation act that funds the Department of Health and Human Services. These legislative mandates may change annually, depending on the priorities of Congress. Compliance with the legislative mandates is mandatory for all PHS awards.
The NIH Fiscal Policies website provides a list of current and historical legislative mandates. While this is an NIH website, the legislative mandates apply to all PHS agencies.
The Acknowledgement Statement is a requirement for many federally funded research awards. When issuing statements, press releases, requests for proposals, bid invitations, and other documents (including conference slides and publications) that describe federally funded projects, recipients must acknowledge the federal government.
Recipients are required to state:
- The percentage and dollar amount of the total program or project costs financed with federal funds
- The dollar amount of federal funds provided to the project
- The percentage and dollar amount of the total costs of the project financed by non-governmental sources
The exact language required for each federal agency may vary, and it is the responsibility of the PI to ensure compliance with the acknowledgement statement requirements for each award. Failure to use the acknowledgement statement may result in a cost disallowance for all costs associated with the publication or document.
Sponsored projects typically require the procurement of goods and services necessary to complete the project’s objectives. Examples of procurement include obtaining supplies, services, equipment, subagreements, travel, and consultants.
All procurement using University funds, sponsored funds, or a combination of both University and sponsored funds must comply with:
- The University of Colorado Procurement Rules
- The Procurement Code of Ethics
- PSC policies and procedures
The PSC provides information on how to purchase specific goods and services on the Commodity Listing website.
All travel-related costs must comply with the PSC Procedural Statement: Travel and follow PSC policies and procedures.
The University’s procurement rules are more restrictive than federal government requirements found in 2 CFR 200. Therefore, all procurement actions must adhere to the University’s procurement rules.
Sponsored funds may be used to obtain equipment and supplies necessary for the successful completion of the project, provided they are allowable under the award terms and conditions. The following graphic identifies the property classifications under 2 CFR 200 and University policy:
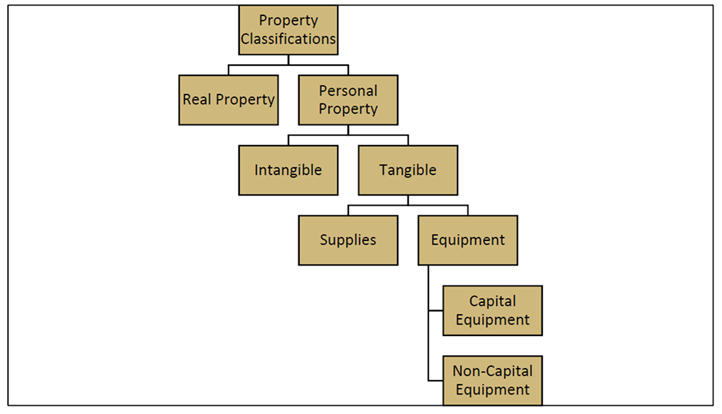
The following list defines the types of property:
- Real property: Defined as buildings or land
- Personal property: Property not classified as real property, including:
- Intangible property: Property having no physical existence, such as intellectual property (trademarks, copyrights, and patents), data and data licenses, and websites
- Tangible property, which includes:
- Equipment: Property with a per-unit acquisition cost of $5,000 or more and at least one year of useful life
- Supplies: Property that does not meet the equipment threshold
Please note that the equipment threshold of $5,000 is based on University policy and must be applied to all federal awards, even though 2 CFR 200 establishes a higher threshold for equipment.
For internal University purposes, tangible property is classified as follows:
- Capital equipment: Property with a per-unit acquisition cost of $5,000 or more and at least one year of useful life
- Non-capital equipment: Property that does not meet the capitalization threshold but is not a consumable item (e.g., a $2,000 laptop)
- Supplies: Consumable items that are disposed of after use
For most federal research awards, tangible personal property (equipment and supplies) acquired with federal funds is classified as exempt property, which provides title of the property to the University and relieves the University of any further obligations to the federal government. For exempt equipment, PIs and administrative units must obtain disposition instructions from the Finance Office. If property is not classified as exempt property, the University only obtains conditional title to the property and must follow all government requirements for use, disposition, and reimbursement as outlined in 2 CFR 200.313 for equipment and 2 CFR 200.314 for supplies.
The University may also obtain and use federal government property under federal awards and subagreements. Government property is property owned by the federal government in the possession of the University for exclusive use on a federal award. Government property may not be used for any other purpose without the sponsor’s prior approval.
PIs and administrative units are required to complete physical inventories of capital equipment and government property acquired with sponsored funds. The frequency of these inventories varies depending on the type of property. The Finance Office will provide administrative units with inventory and property reports to facilitate the physical inventory.
All property obtained through University funds, sponsored funds, or a combination of University and sponsor funds belongs to the University. PIs and administrative units may not use property for non-work-related purposes. All University property must be returned upon employee termination or when the property is no longer required for official business. An administrative unit has the right to retain all property obtained under a sponsored project when the PI transfers institutions.
All University employees must comply with the University’s University’s Capital Equipment and Government Property policy and the University’s Inventories Policy.
Most sponsors require progress reports that detail the project’s accomplishments and identify corrective actions to address any problems, delays, or adverse conditions in meeting the project’s objectives. The award terms and conditions will specify the reporting requirements. PIs and their administrative units are responsible for writing and developing progress reports and ensuring that all sponsor deadlines are met. Inadequate or late progress reports may result in delays in future funding and possible award suspension or termination.
Federal research awards generally require the submission of the Research Performance Progress Report (RPPR). The specific requirements for the RPPR vary among federal sponsors. The RPPR is used as a non-competing continuation application, which releases funds for the next budget period within the period of performance.
For NIH awards, the RPPR is completed and submitted through eRA Commons. As the NIH RPPR requires AOR submission, only OGC may submit the NIH RPPR. Additional information regarding University requirements for NIH RPPRs is available on the OGC website.
The submission requirements for other sponsors will vary. PIs and their administrative units must ensure that OGC Pre-Award provides any required AOR signature or submits any progress report requiring AOR submission.
Sponsors provide award amendments to the original award to allocate funding for subsequent budget periods, provide supplemental funds, or update the terms and conditions of a project. PIs and their administrative units must take the following steps when receiving an award amendment:
- If the amendment needs to be signed, complete the Contract Amendment Form, or
- If the amendment does not need to be signed, email the amendment and Master Proposal/PeopleSoft Contact Number to [email protected].
- Note: State Option Letters do not require signature but should be routed using the Contract Amendment Form
Sponsors may monitor awards through a variety of activities, including:
- Reviewing and approving progress reports
- Financial inquiries
- Financial reporting
- Requesting invoice documentation for submitted expenses
- Certifications and representations
- Desk reviews
- Site visits
- Audits
PIs and their administrative units are responsible for complying with all sponsor requests in a timely manner. Fiscal Compliance is the coordinating office for all site visits and sponsor audits. PIs and their administrative units should immediately notify Fiscal Compliance when a sponsor indicates they are planning a site visit or audit of a sponsored project.
The University’s Record Retention Matrixgoverns the record retention requirements for all University-related paperwork, documents, and files, including those for sponsored projects. The Record Retention Matrix is based on the University of Colorado’s APS Retention of University Records policy. For federal sponsored projects, the University’s record retention requirements are more restrictive than 2 CFR 200. Therefore, PIs and administrative units must follow the University’s policy instead of the federal requirements. Failure to comply with the University’s record retention policies may result in audit findings and questioned costs.
Office of Grants and Contracts
CU Anschutz
Fitzsimons Building
13001 East 17th Place
W1126
Aurora, CO 80045
303-724-0090
Financial Services
Leadership
- Executive Vice Chancellor for Administration and Finance | CFO, Terri Carrothers
- Vice Chancellor for Research, CU Anschutz, Thomas Flaig, PhD
- Associate Vice Chancellor for Research and Chief Research Officer, CU Denver, Philip De Leon, PhD
- Associate Vice Chancellor for Financial Service and Controller, Amy Gannon
CMS Login