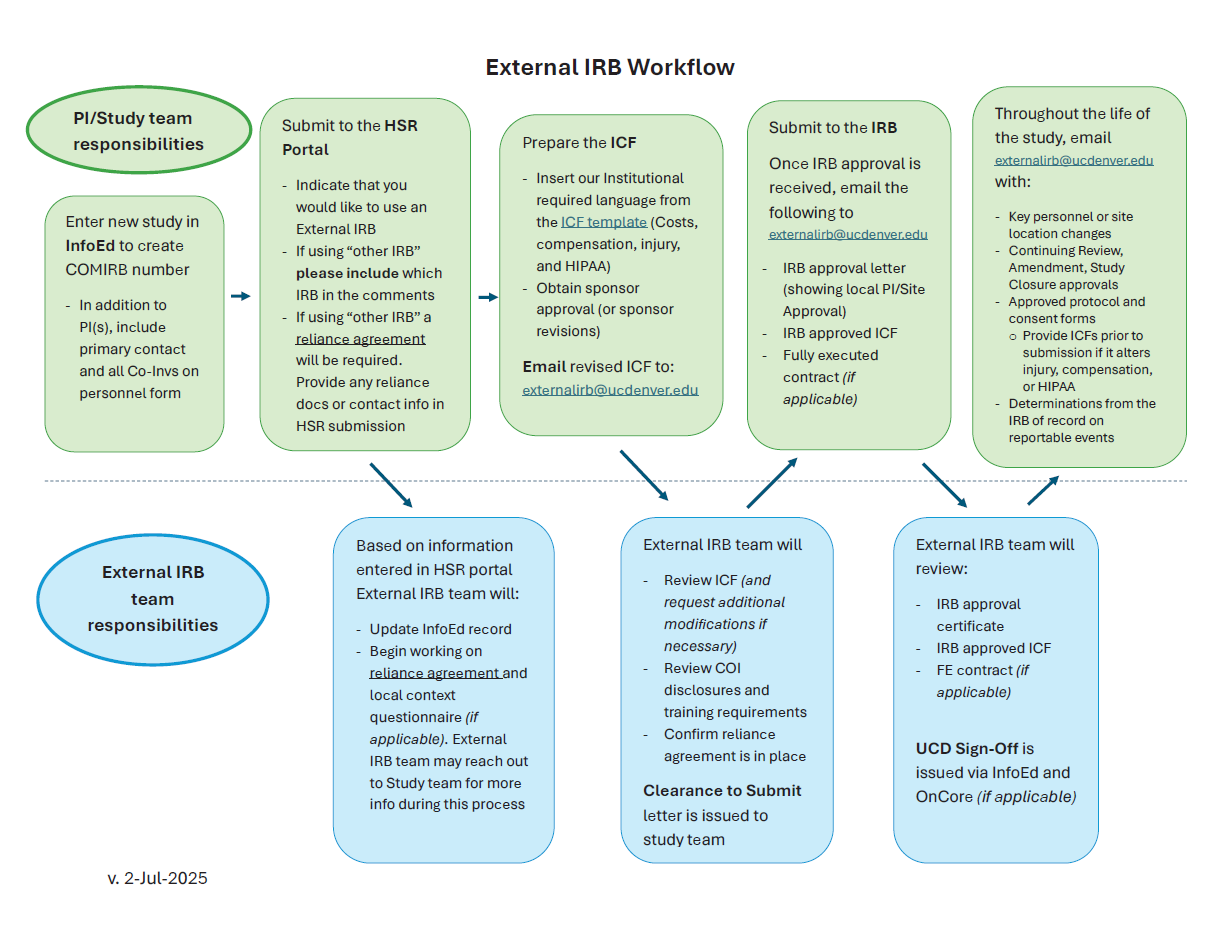
External IRB Overview
This guidance addresses the process for ceding IRB review to an outside IRB, and ongoing responsibilities for these studies. If you want to use COMIRB as your IRB, contact the COMIRB helpdesk at [email protected]
The External IRB Team works within COMIRB here at CU Anschutz, this team was previously within Clinical Research Administration.
Our work involves managing all protocol documents and information related to human subject research protocols submitted to commercial IRBs (i.e., WCG, Advarra, NCI Central IRB, etc.) and non-commercial IRBs (i.e., academic institutions). We are required to sign off on several items before you can submit your application to an external IRB.
All protocols using an external IRB must be submitted through the HSR Portal by filling out the Protocol Assessment Form.
Please note: If your study is deemed exempt or does not meet the definition of human subjects research, we are not allowed to rely on an external IRB. Additionally, unfunded studies may not rely on a commercial IRB.
This page is the condensed process, for a more detailed process explanation navigate to the External Submission Guide page.
If you want to use COMIRB as the single IRB contact the COMIRB helpdesk at: [email protected].
Once a Reliance Agreement is established and the External IRB Team has provided both Clearance to Submit and UCD Sign off for the duration of the study, please notify the External IRB Team via email at [email protected] of the following:
| Your responsibilities | What we check |
| Maintain IRB approval from the outside IRB for the lifetime of this research project | Continuing review and amendment approvals and study closure |
| Communicate changes in key study team members: PI, Co-Investigators, and Primary Contact or site locations | Confirm current COI disclosure and up-to-date CITI training |
| Provide documentation regarding revised consent forms | Checking for approved injury, cost, compensation, and HIPAA language. |
| Provide determinations from the reviewing IRB for reportable events | If it is necessary to provide additional support to the local research team |
Standing Agreements (WCG/Advarra/NCI)
Our institution maintains standing agreements with WCG, Advarra, and the NCI Central IRB (NCI CIRB) to facilitate efficient review and oversight of eligible studies. These agreements allow us to rely on these external IRBs as the IRB of record, streamlining the review process for multi-site and industry-sponsored research.
Smart IRB Acknowledgment
SMART IRB (the Streamlined, Multisite, Accelerated Resources for Trials IRB Reliance platform) is designed to harmonize and streamline the IRB review process for multisite studies, while ensuring a high level of protection for research participants.
SMART IRB is not an IRB; rather, it's a platform that offers a master IRB reliance agreement (the SMART IRB Agreement) and a web-based system (SMART IRB's Online Reliance System) that provides a central process for participating institutions and their investigators to request, track, and document study-specific reliance arrangements. Investigators and their study teams, together with institutional and HRPP/IRB offices, use the SMART IRB platform to initiate single IRB review of a study. COMIRB is a member of the SMART IRB system.
The agreement must be routed through the external IRB team office for signature.
IREx
IREx is a web-based platform that streamlines the single IRB review process for multicenter studies. It helps institutions manage reliance agreements, track local context information, and share review documentation between the reviewing and relying IRBs. Some outside IRBs will choose to use IREx, in these instances the study team will have access.
IRB Authorization Agreement
This agreement will be used for institutions that are not part of the SMART IRB network. It lists responsibilities for both the reviewing institution and the relying institution. The agreement must be routed through the external IRB team office for signature.
Local Context Forms
For studies reviewed by outside IRBs without a standing agreement, COMIRB provides local context forms to ensure institutional requirements and site-specific considerations are addressed. Investigators, their study teams, and institutional and HRPP/IRB offices work together to complete these surveys.
I have received a local context form; can I fill this out?
A: Yes, but you need to send it to us for final review updates and sign-off.
If we have a reliance agreement in place is that the same as IRB approval, and can we begin enrolling?
A: No! The reliance agreement is the agreement between the institution and the reviewing IRB. IRB approval is achieved by the reviewing IRB, approving us as a site.
My sponsor has asked that we use WCG or Advarra IRB, is this ok and how do I get started with my submission?
A: Yes, this is ok. See our process guide for submission details.
We’ve been asked to participate as an additional site on an ongoing trial. The study is already approved by an outside IRB. Who do I contact to rely on that IRB?
A:Send any questions to [email protected]
I have personnel updates to make, what do I do?
A: Email any changes to key study personnel (PI, Co-Investigators, or Primary Contact) to [email protected]
Do I submit my subject recruitment materials to the COMIRB when using an external IRB?
A: No, not all subject materials are necessary to send, but send initial approval letter or amendment approval to [email protected]
Do I submit my SAEs or UAPs to the COMIRB when using an external IRB?
A: Do not send these to COMIRB, but yes send them to your reviewing IRB and send those determination letters to [email protected]
Can I change IRBs?
A: Yes, simply email [email protected] and we will help you navigate the process.
If my study is federally funded, does the one-time $5,000 fee still apply?
A: No, and fee waiver forms are no longer required.
Why are there two “signoffs”?
A: There are not two signoffs, but there are two stages to approval with one signoff. First, clearance to submit is the process that once attained is your green light to submit to your IRB of record. Second, is UCD sign-off, which is your official IRB approval from us.