ClinicalTrials.gov Tip of the Week: Seife et al. v. HHS et al.: Results Must be Submitted for “pACTs” to Clinical Trials.gov
Aug 20, 2020|
A federal court has ruled that FDAAA unambiguously requires responsible parties to submit Basic Results data to ClinicalTrials.gov for pre-Final Rule, pre-approval Applicable Clinical Trials. The ruling by a federal judge set aside a previous HHS interpretation of the Final Rule (42 CFR Part 11). Responsible parties must now submit results data to ClinicalTrials.gov for any Applicable Clinical Trial (ACT) that:
Also, please note that:
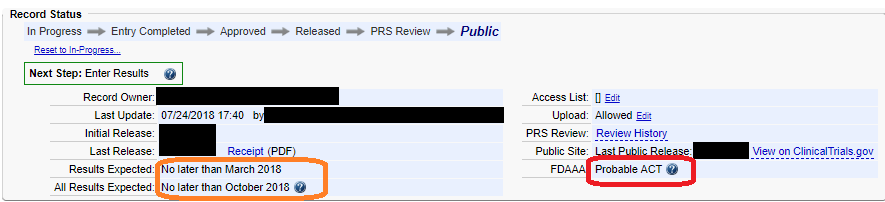
What does this mean for CU Responsible Parties? It means that if you are a Record Owner for any ClinicalTrials.gov records, even for older studies, you should log into ClinicalTrials.gov and review your records to verify whether you need to submit results. ClinicalTrials.gov’s PRS system treats these “probable Applicable Clinical Trials” or “pACTs” identically to post-Final Rule ACTs when flagging records for required results submission. If results for a pACT are overdue, you would currently see a ‘Late Results per FDAAA’ problem message on the record. The Clinical Research Support Center also treats pACTs just like ACTs. Record Owners with “Late Results” for pACTs are notified of their status by the PRS administrator and prompted to submit results data.
To view your ClinicalTrials.gov records and check whether your study is a pACT/ACT:
 ______________________________________ The Clinical Research Support Center is here to help! If you need assistance submitting results, accessing your records, or understanding the requirements for your studies, contact clinicalresearchsupportcenter@ucdenver.edu. We will work with you and your team to answer your questions and help you bring your record back into compliance. |