HSR Initial and Amendments Portal
Human Subject Research Portal
Before you start, you will need:
- IRB (COMIRB) Tracking Number – to obtain a COMIRB number (24-XXXX), select CU Denver from the InfoEd eRA login page. Then, click Human Protocol, and Create New Human Protocol, do not click submit.
- Protocol (draft is acceptable)
- Consent (for studies that have patient interaction; draft is acceptable)
- Draft contract (if industry funded/industry initiated)
- Calendar of Events (draft is acceptable)
- You’ll be able to include other optional documents to help us review your study.
IMPORTANT: COMIRB requires a Portal Clearance Memo with your submission for initial review of non-exempt research.
Enter your email address when prompted, and you’ll receive the memo from [email protected] once the review is complete.
**Please make sure to check your Inbox and save this email as Clinical Research Administration will not be copied.**
CHCO only studies: Portal Clearance Memos not required; provide your CHCO approval letter to COMIRB instead.
If you have received a Just-In-Time (JIT) notification from the NIH for IRB approval, submit your study to COMIRB first. Submit your study materials to the HSR Portal promptly after COMIRB approval.
Investigator initiated studies with no previous scientific review:

If your study is investigator initiated, locally written with no previous scientific review, it will require either PRMS (if oncology) or SARC (all other non-cancer studies) review to receive portal clearance.
For Children’s Hospital Colorado studies:
You must complete the CHCO ancillary form, as part of the portal submission process. COMIRB does not require Portal Clearance Memos for CHCO only studies. You will provide them your CHCO approval letter instead.
Need to save and come back to your submission?
You have the option to save and return later if you start the form and need to some back. Just write down your return code!
You can finish your submission later on if you are not ready by clicking Save & Return Later at the bottom of the submission page:

This will provide a return link and code. Please only use this if you have not fully submitted your portal form. If you need to return and make corrections to an already submitted study, please request the code and link from OnCore Support ([email protected]).
Using the return code and link, you will be given two options after you click the link:
- Submit a return code
- Start over
If the “start over” button is selected, it will remove all responses from the form. Please use the option only when you need to delete every entry from your portal survey.
NOTE: Submissions started but not completed within 90 days will be deleted.
1
Study Information Section
Please ensure that your COMIRB number is accurate:

2
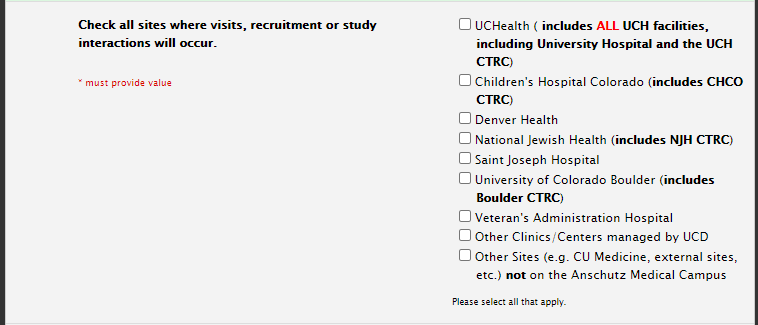
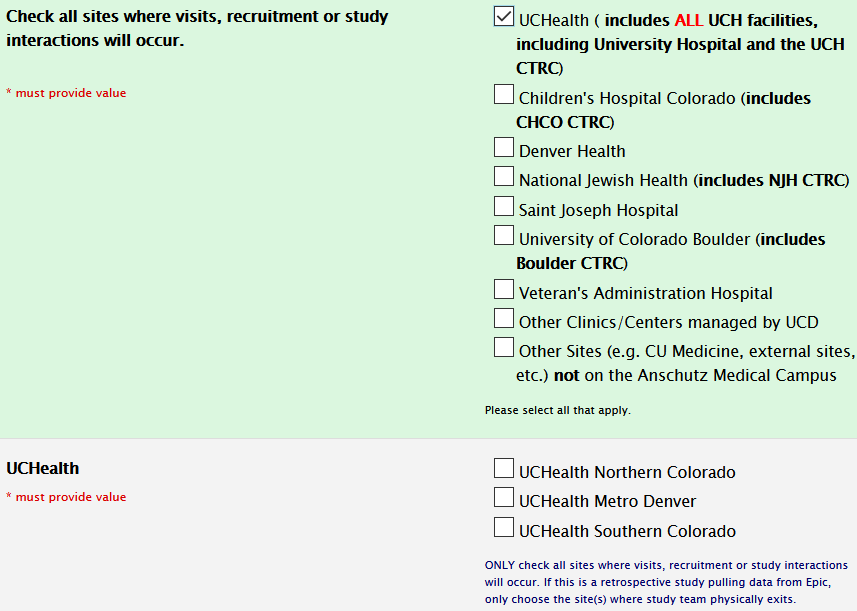
Study Information Section: Sites
When adding sites, only include sites where recruitment/patient interaction is involved:

3
Sites: Retrospective Studies
For a retrospective study pulling data from Epic, only choose the UCHealth site(s) where the study team physically exists.

4
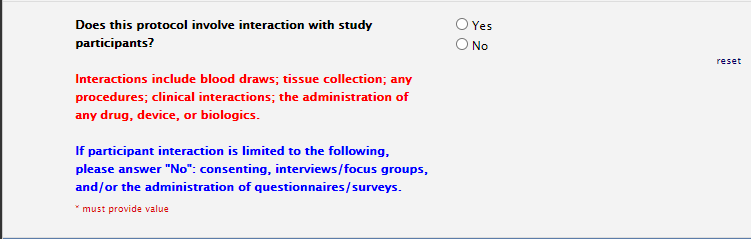
Study Information Section: Patient Interaction
If you anticipate any invasive and/or billable procedures at any of the hospitals, please select yes to patient interaction.

5
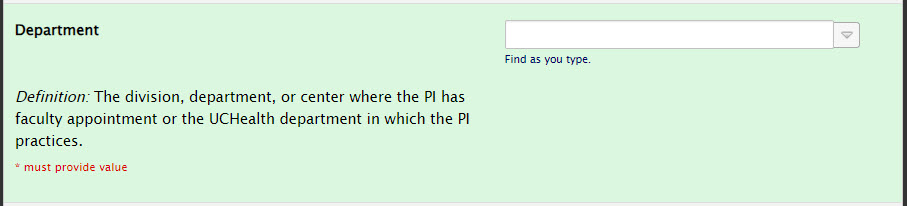
Principal Investigator Section: Department
Please choose the correct division, department, or center where the PI has faculty appointment or the UCHealth department in which the PI practices:

6
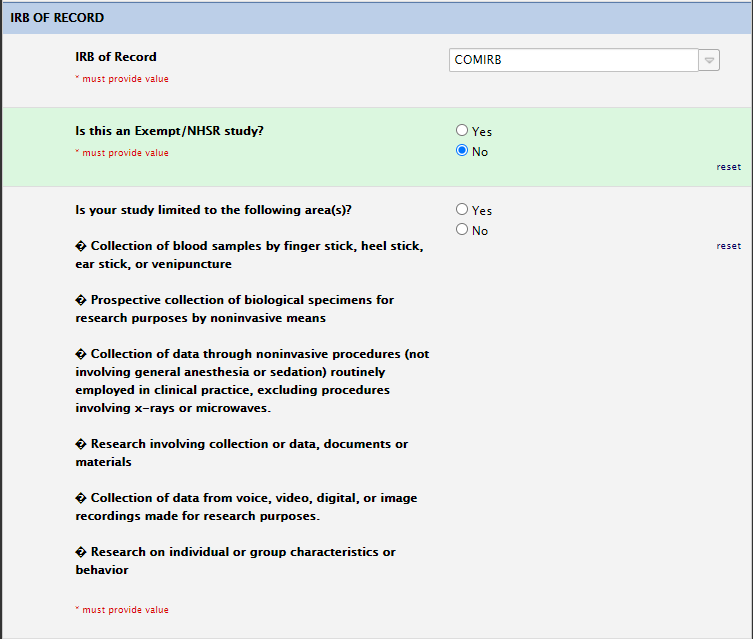
IRB of Record Section
For COMIRB studies, only choose exempt if you anticipate exempt review from COMIRB. Please review the “is your study limited to the following area(s) section. This indicates whether or not this will go to COMIRB expedited or COMIRB full board:

7
Staff Section
When entering staff, please note we only need local UCD, DH, CHCO and/or UCH staff. Do not enter collaborators at institutions other than these. Additionally, please do not select “Other” as a department.

8
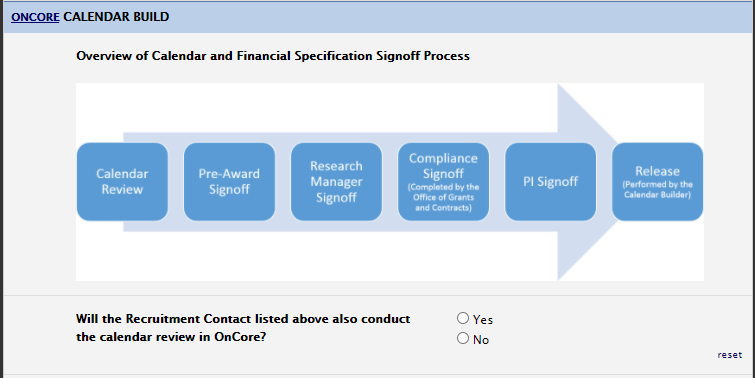
Calendar Section
Please ensure that the primary contact listed in staff section will be doing the calendar signoff. If not, please indicate in the Calendar Build section. Please note that a calendar will be required for any study that has invasive/billable procedures.

9
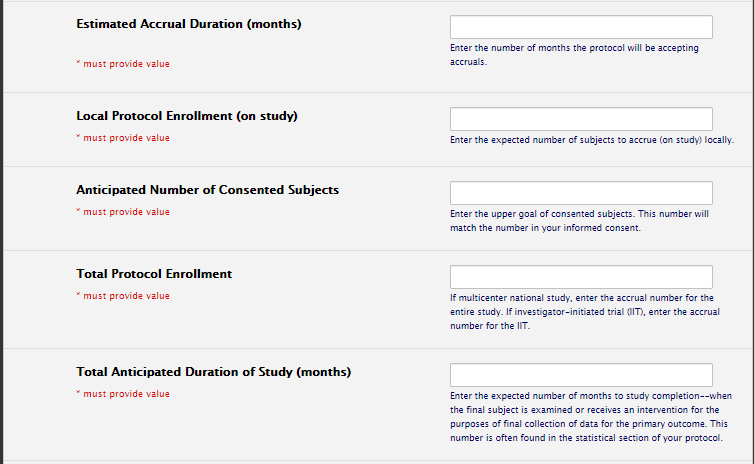
Feasibility/Recruitment Plan Section
Accrual numbers should line up. If a multi-site trial, Total Protocol Enrollment should be larger than local protocol enrollment. Typically, anticipated number of consented subjects is also larger than local protocol enrollment, or the same number:

10
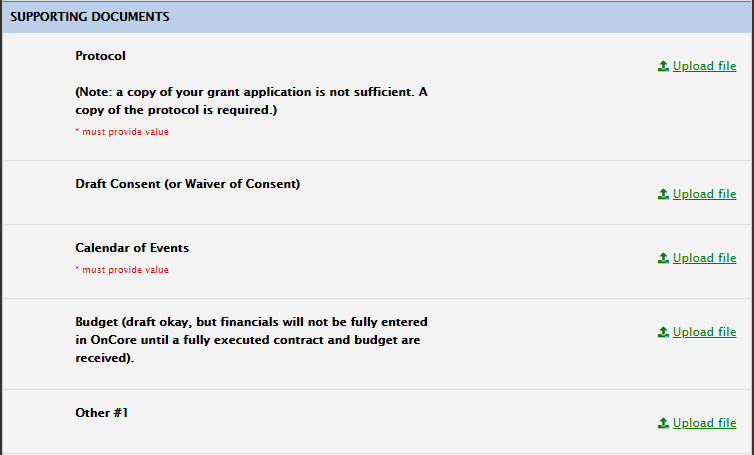
Supporting Documents Section
For supporting docs, please note that only the fields with '*must provide value' are required to be uploaded. The others are optional.

11
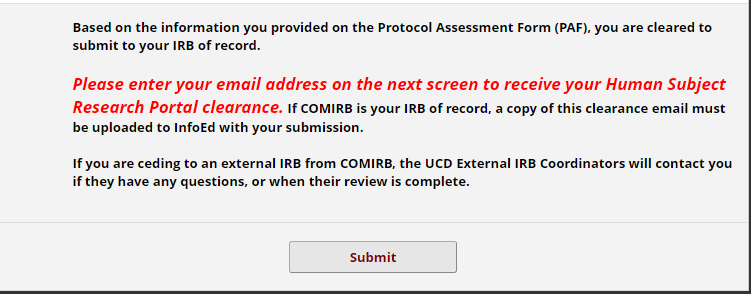
Submission: Final Steps
When you submit, please enter an email on the following page after submission so that you receive your portal clearance.

Need more specific guidance?
1
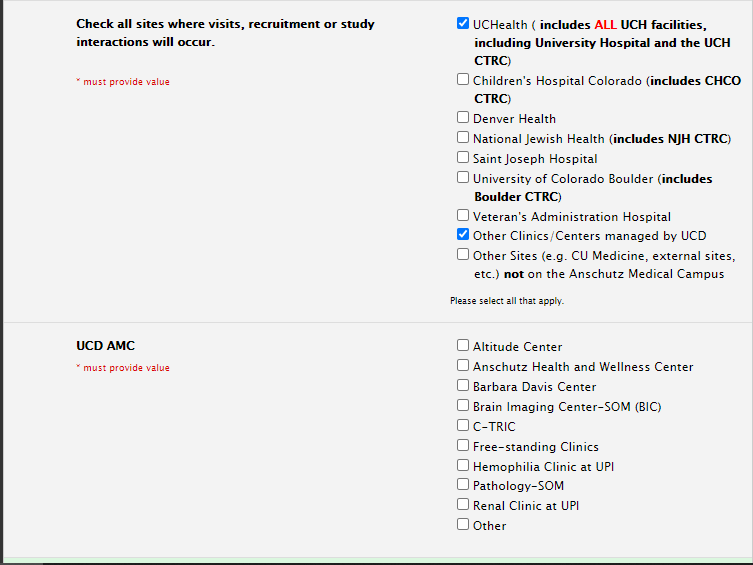
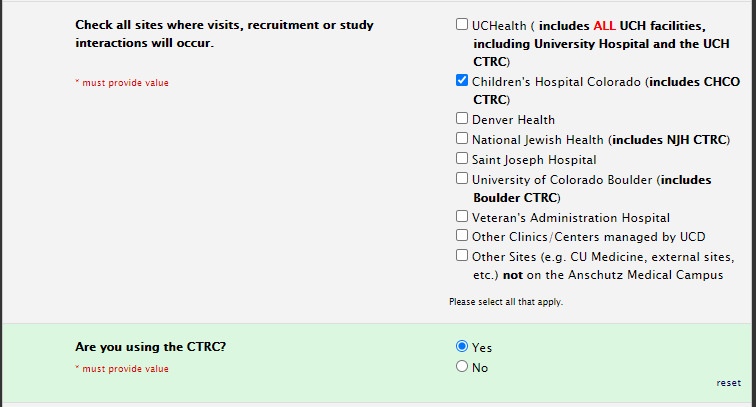
Selecting Sites
Will your study include UCD clinics/centers? If so, select “Other Clinics/Centers managed by UCD” to open the UCD AMC list where you can indicate the specific sites.

2
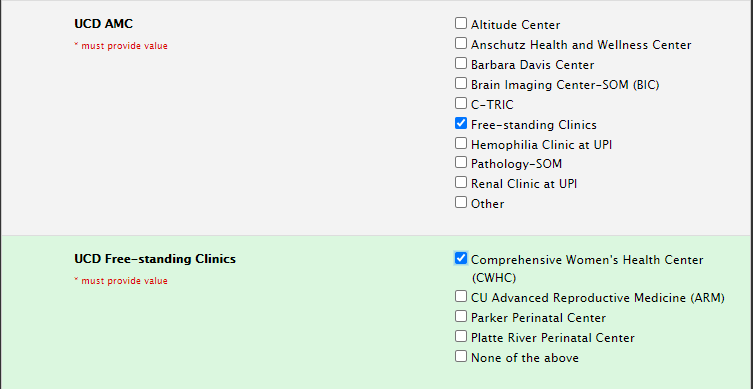
Selecting Sites: Free-standing Clinics
If you are using the Comprehensive Women’s Health Center, CU Advanced Reproductive Medicine, Parker Perinatal Center or Platte River Perinatal Center sites, please select free-standing Clinics as indicated below:

3
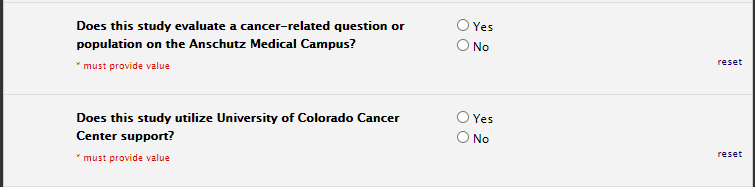
Oncology
If any cancer population is included on study, please select yes to cancer related questions:

4
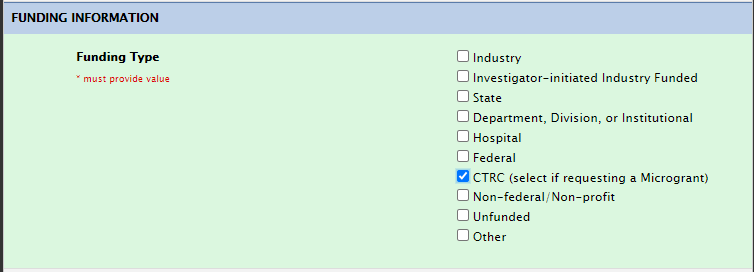
Funding Section: CTRC
For microgrants, please select microgrant under funding:

5
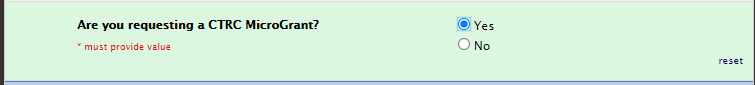
CTRC MicroGrant
There is also an additional question in this section pertaining to microgrants that need to be selected as yes:

6
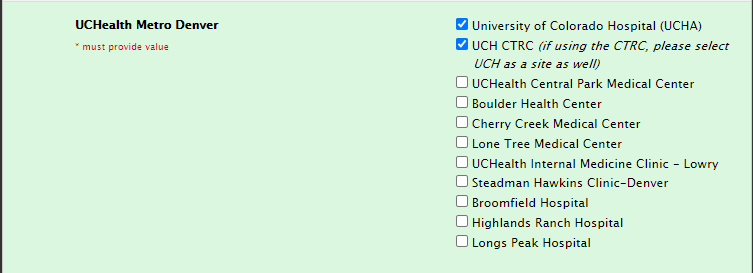
Site selection for CTRC usage
Also, please select UCH as a site if you are utilizing the CTRC at UCH.

7
CHCO CTRC
If you are using CHCO CTRC, please select yes to this question in the site selection section:

8
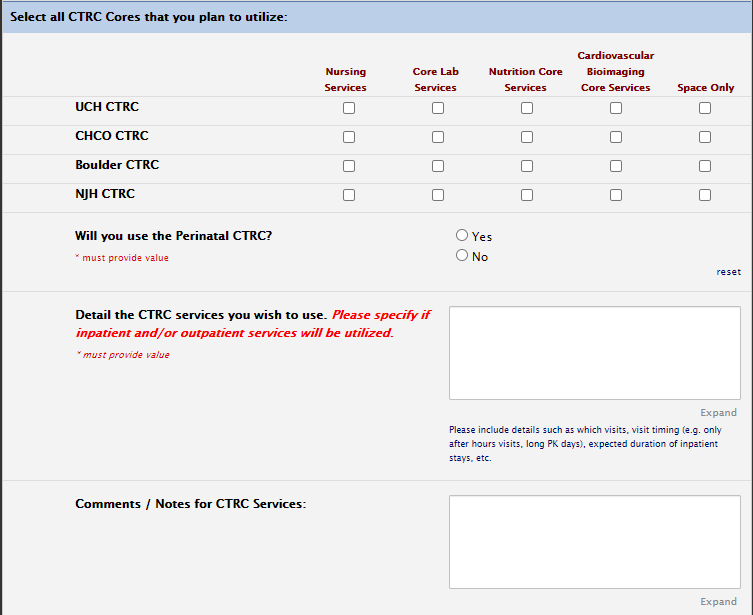
CTRC services
You must also fill out the CTRC section for services you intend to use:

9
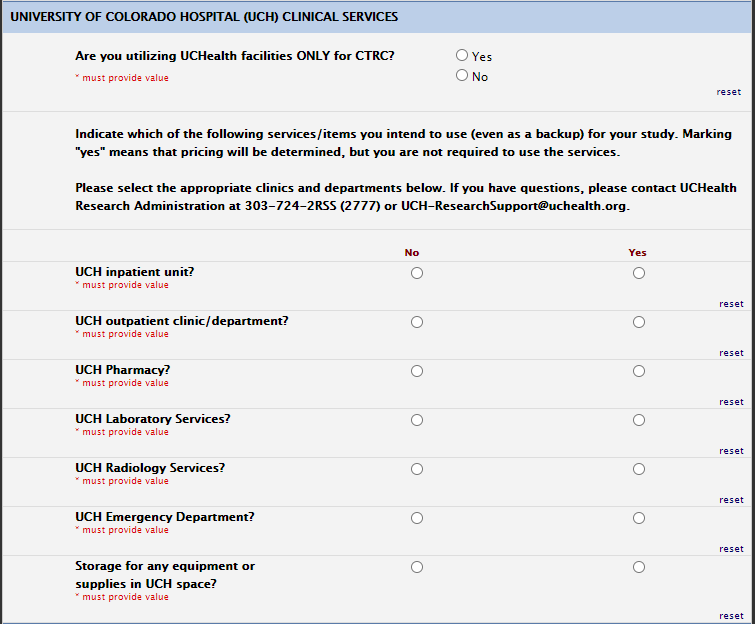
UCH Services:
If you plan to use the CTRC in addition to UCH services, please select no to the following question and fill out the UCH services you intend to use. Please select yes if you only intend to use UCH for CTRC services on the first question above and select all UCH services as no.

10
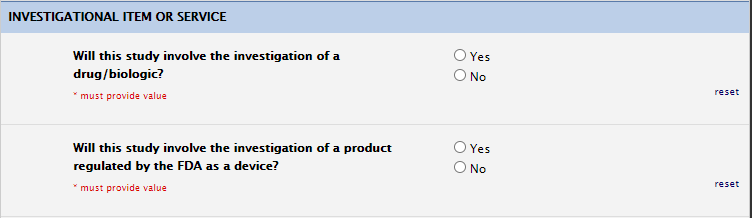
Investigational Item or Service
Please ensure these questions are answered per the protocol. When applicable, further information on investigational drugs (i.e., IND number) and devices (i.e., IDE number) may be requested. If your team is also planning on managing/storing drugs and/or devices yourself, we need this indicated in these questions.

After you submit to the Amendment Portal, Clinical Research Administration and OnCore Support teams review the changes for regulatory and billing compliance implications.
Information for Sponsors
The University of Colorado has developed a partially executed CDA (Confidential Disclosure Agreement).
If your sponsor can accept this document by including legal address, effective date and signing, the CDA will be fully executed and may be used. We have instructed our study teams how to work with you on the use of this document.
This partially executed CDA will decrease completion time for a fully executed CDA.