OGC News - January 2022
Financial Services Connection
Jan 14, 2022
CONTRACTING SERVICES
New & Improved Website Content for OGC Agreements
The OGC Contracts team has made many additions and improvements to our website. New content can be found on the following topics:
- New FAQ section for sponsored project agreements and fee for service revenue agreements
- Updated Subcontract process documents & materials
- DocuSign Process & Instructions
- Intercampus Subcontract (task order) Process
- Updated Attachment 3B for incoming FDP template agreements
- Research Services Agreements & Practice Participation Agreements
Sponsored Projects: Contract v. Amendment Routing Reminder
 All new agreements for sponsored projects should be routed in InfoEd. Please use the OGC continuation formstack request link only for amendments or modifications to existing agreements that have an existing InfoEd proposal number.
All new agreements for sponsored projects should be routed in InfoEd. Please use the OGC continuation formstack request link only for amendments or modifications to existing agreements that have an existing InfoEd proposal number.
A contract is a legally binding agreement between two parties. Anything that binds the University to certain terms and conditions is considered a contract. Once signed, this formalizes the terms between the University and external party to start the project.
An amendment changes an existing contract by adding new terms and conditions, changing existing terms, changing the scope of work details, adding new money, or changing the duration of the agreement. Any amendment will be processed by the same office that is responsible for processing the original agreement.
Updates to the InfoEd Proposal Routing Form to COI/HSR
First, two questions related to Institutional Conflict of Interest have been added:
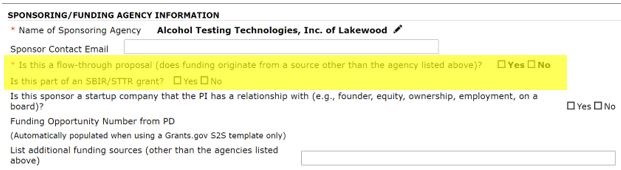
These two questions will help our COI office have notice when a COI management plan may be needed, as well as inform the OGC Contracts team what type of contract may be needed.
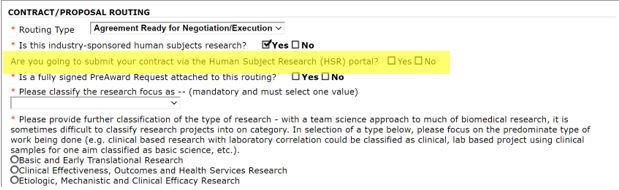
Second, a question related to HSR submissions has been added. This question is intended to help ensure that Industry funded human subjects research contracts that may not be routed via the HSR portal are reviewed by the CRAO office.
New Field in OGC Continuation Formstack
In an effort to improve communication between our OGC Contracts team and our departmental business partners, we have recently added two new fields to our amendment formstack request:
- Deadline field- a new optional field to input any related deadlines associated with the amendment’s review that we should know about in advance.
- Rush Request- a new optional field. If you select yes, a mandatory field will appear for you to include details related to the reason for the rush request. Please use this rush option only for situations that are truly an emergency, such as the risk of losing funding if the amendment is not returned by a particular date. You are also welcome to email ogc.contracts@ucdenver.edu in these situations so we can ensure prompt review and return of the amendment.
Updated FDP Templates for Outgoing Subawards
The OGC Subcontracts team has updated our FDP subaward templates to align with the most recent changes published on the FDP website. This includes a new Attachment 7 to govern data transfers incoming or outgoing related to the subaward, where applicable. In most cases, this will replace the need for a separate Data Use Agreement associated with the subaward. This will also bring our campus into alignment with the process many other Universities have adopted and should streamline the outgoing subaward negotiation process.
New Sub-recipient Commitment Form for Outgoing Subcontracts
There is a new Sub-recipient Commitment Form available in the Process Documents section of our OGC Subcontracts website. This new version allows members of the FDP Clearinghouse to opt out of several questions within the form and should expedite the form’s return going forward.

TRAINING & COMPLIANCE
Financial Services Sponsored Project Training Program - Spring 2022
 Financial Services is pleased to announce that registration for the sponsored projects training courses for spring 2022 is now open in SkillSoft.
Financial Services is pleased to announce that registration for the sponsored projects training courses for spring 2022 is now open in SkillSoft.
The courses will be offered via Zoom and are open to all employees. These courses are particularly recommended for new employees at the University and to employees new to sponsored research.
The courses for spring 2022 are:
- SP 1 – Introduction to Sponsored Projects
- SP 2 – Cost Principles for Sponsored Projects
- SP 3 – Pre-Award Administration for Sponsored Projects
- SP 4 – Post Award Administration for Sponsored Projects
- SP 11 – Contracting for Sponsored Projects
- SP 12 – Introduction to 2 CFR 200 (Uniform Guidance)
- SP 13 – Introduction to the NIH Grants Policy Statement (NIHGPS)
- SP 16 – Subrecipient Monitoring Policies and Procedures
Registration, course descriptions and time and date offerings for each course is found on SkillSoft.
In addition to these courses, Financial Services is also offering the following finance classes:
- Financial General Ledger, offered on January 21, 2022 and March 11, 2022
- Finance Inquiry, offered on January 14, 2022 and March 4, 2022
Completion of both finance courses are required before an employee may complete and submit journal entries. If you have questions about how to register, you can visit the Skillsoft Help Page. Under the Resource Tab, there are a number of resources and quick reference guides. In particular, the Instructor Led Training (ILT) guide covers how to enroll in an ILT course, how to check your enrollment status, and how to withdraw from an ILT course. Enrolling in Instructor Led Training may be helpful as well. Finally, under the Instructor Led Training tab, there is information about where to find instructor led training (ILT) courses. Additionally, individual or small group trainings are also available upon request
Please feel free to email Shane Jernigan if you have questions about the sponsored training program. Questions regarding the finance courses may be submitted to finance.access@ucdenver.edu.
Certified Research Administrator (CRA) Exam Group Study Sessions
The CRA exam testing window for Spring 2022 is May 14-28. To assist employees in preparing for the exam, Financial Services is offering exam preparation study sessions starting in February. The study sessions include 12 one-hour reviews that cover the exam’s Body of Knowledge. The sessions will be held on Fridays at 10am-11am on Zoom.
Anyone interested in participating in the group study sessions may contact Shane Jernigan for additional information. Additional information about the CRA exam can be found on the Research Administrators Certification Council website.
NIH Virtual Grants Seminar
In November, NIH hosted a seminar on Program Funding and Grants Administration. Over 15,000 people participated to some extent. Some of our Office of Grants and Contracts team members attended and wanted to share some of the great presentations. If you are new to NIH awards or need a refresh on a specific topic or category, please visit our OGC Training Website for an external link to the presentation materials and videos that were shared.1099 Reporting
 As a reminder, 1099 reporting was due to the PSC on January 6, 2022. If you paid a study subject participant greater than $100 in the calendar year (between January 1, 2021 – December 31, 2021), and not have not already completed the 1099MISC Spreadsheet, please do so IMMEDIATELY. Once completed, please send the 1099MISC spreadsheet via SECURE/ENCRYPTED email to Mai Ngo and PCGC.
As a reminder, 1099 reporting was due to the PSC on January 6, 2022. If you paid a study subject participant greater than $100 in the calendar year (between January 1, 2021 – December 31, 2021), and not have not already completed the 1099MISC Spreadsheet, please do so IMMEDIATELY. Once completed, please send the 1099MISC spreadsheet via SECURE/ENCRYPTED email to Mai Ngo and PCGC.
Additionally, January 1, 2022 marks the start of a new 1099 reporting period. Please ensure that tracking spreadsheets for cash or gift card payments to study subjects and other participants is updated to track a new year. W-9 collection should be conducted when an individual receives greater than $100 in a calendar year. If you have questions on this process, please contact PCGC.

BILLING & ACCOUNTS RECEIVABLE
Fun Facts
The Office of Grants and Contracts processed a total of 9,274 invoices in 2021, billing over $151 million dollars. With the implementation of Billing Automation, 3,828 of these invoices were able to utilize that functionality, preserving approximately 64 hours to focus on more complex issues. For more information about the billing automation initiative, please see the controller’s innovation award site.
Cash Receipt Form (update)
There is a new version of the Cash Receipt Form to be used when depositing gift funding, effective 12/1/2021. To deposit gifts, use the Cash Receipt-Gifts form instead of the Cash Receipt form, and take the form to your campus Advancement Office for deposit to a gift fund.

POST AWARD
Timeliness of Journal Entries for Sponsored Projects
 Reminder - If a journal entry is moving an existing expense to a sponsored project, it is a cost transfer and must contain supporting documentation to meet the requirements of the cost transfer policy. One of the requirements of this policy is timely corrections.
Reminder - If a journal entry is moving an existing expense to a sponsored project, it is a cost transfer and must contain supporting documentation to meet the requirements of the cost transfer policy. One of the requirements of this policy is timely corrections.
In order to demonstrate when the original charge occurred, the journal must include original details of the charges in the journal attachments. In addition, cost transfers that are being completed over 90 days from the original charge should sufficiently explain why they are older than 90 days, and what will be done in the future to ensure cost transfers occur timelier.
When creating journal entries, please ensure that financial details are included and specific charges are indicated. If over 90 days from original charge, please indicate the additional requirements in the Journal Description. If the field is not long enough to demonstrate the justification you can include a note or word document as an attachment.

SPONSOR UPDATES
Updated NSF Pre-Award and Post-Award Disclosure Table
NSF has issued an updated version of the table entitled NSF Pre-award and Post-award Disclosures Relating to the Biographical Sketch and Current and Pending Support. The updated table dated January 10, 2022, includes information regarding start-up packages provided by proposing organizations as well as outside organizations. Any questions regarding the contents of the table should be directed to the Policy Office at policy@nsf.gov.
Updated NIH Grants Policy Statement Released
The NIH has released an updated version to the NIH Grants Policy Statement (NIH GPS). The December 2021 version governs all NIH grants and cooperative agreements with budget period beginning on or after October 1, 2021. A list of significant changes to the NIH GPS can be found here.
NIH eRA Commons ID Requirements on/after 1/25/2022
NOT-OD-21-109 NIH, requires all individuals listed on the R&R Senior/Key Personnel form to have an eRA Commons ID.
- This new requirement applies to all Senior/Key Personnel and Other Significant Contributors proposed in NIH applications.
- Missing Commons ID will cause an ERROR beginning 1/25/2022 at submission and will prevent proposal submission.
- CU Denver will create Commons IDs for non-CU personnel, but we ask for a good faith effort on the part of the non-CU personnel/institution to register and obtain a Commons ID at their local institution first time permitting.
NIH Forms G Updates – Proposals Due on/after 1/25/2022
NIH will require use of FORMS-G forms/applications for grant application due dates on or after 01/25/2022. NIH’s High-Level Form Change Summary is available here. A summary of some changes and links to details include:
Biosketch
Use of new Biosketch templates will be required, with the following changes to the requirements:
- Two options for creating a NIH Biosketch: SciENcv (recommended) or Word template. Instructions, FAQ, samples and the current Word templates are available here.
- Section A: Applicants may choose to include details on ongoing and completed research projects from the past three years that they want to draw attention to. Formatting samples provided in NIH’s sample biosketch.
- Section B: Section B has been renamed ‘Positions, Scientific Appointments, and Honors’.
- For foreign appointments, supporting documentation will need to be provided at the Just-In-Time (JIT) phase as detailed under Other Support.
- Section D: “Research Support” removed for non-Fellowship Biosketches. Included on Fellowship Biosketches as “Scholastic Performance”.
To subscribe to the GC-Updates list serve, click here.