Why was I notified that my record has errors?
Oct 16, 2020Have you received a message that your ClinicalTrials.gov record needs attention, but everything was fine the last time you checked? The problem might be due to your Study Status module being out of date.
The Study Status module contains the time-sensitive information you entered about when your study starts, completes, and about the current status. It also must be updated at least once every 12 months. The image below shows an up-to-date study status module.
When the study status information gets out of date, for example if the “anticipated” study start date has occurred and is now in the past, but still listed as “anticipated”, the record will start to generate errors. Below is a study status module where both the “anticipated” study start date is in the past, and the record status is also still “not yet recruiting”.

The longer the record goes without being updated, the more errors will be generated.
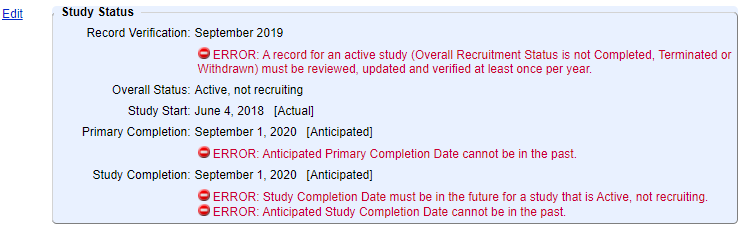
Think of your study status section as your canary in a coal mine, reminding you to regularly check and update your record in order to save yourself a lot of potential trouble later.
To update the Study Status section and clear the errors:
- Log into ClinicalTrials.gov (UserID = your email, Organization = UColorado)
- Open the record. This will take you to the Record Summary page
- Click “Open” next to the Protocol Section
- Click “Edit” next to Study Status
- Update the information and save
- Don’t forget to go back to the Record Summary page and click the “Complete” button when finished.
Tags:
clinicaltrials.gov
completion date
errors
study status