When is a ClinicalTrials.gov Record Considered "Complete" or "Closed"?
Feb 3, 2022A ClinicalTrials.gov record is considered closed when the following are true:
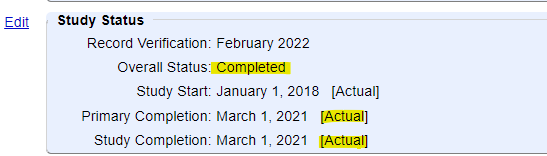
1) Under the Study Status module:
- The Overall Study Status is "Completed", "Terminated" or "Withdrawn"
- The Primary and Study Completion Dates are "Actual" (rather than "Anticipated"
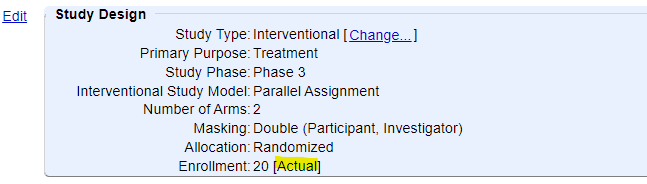
2) Under the Study Design module, the final Enrollment has been entered and changed to "Actual" (rather than "Anticipated").
3) Any required documents have been uploaded to the Documents section:
- If results are required, the Protocol and Statistical Analysis Plan must be uploaded (usually, the SAP is included in the protocol).
- If the study is an Common Rule agency-supported clinical trial, the Consent Form must be uploaded.

4) If results are required, results data has been submitted, passed QC review, and been made public with no outstanding Major Comments.

For help closing out your ClinicalTrials.gov records, contact [email protected].
Tags:
clinicaltrials.gov
closed
Documents
results
status