The Sponsors and Collaborators Module
Sep 30, 2021The Sponsors and Collaborators module is part of the Protocol section of your ClinicalTrials.gov record. There are two required fields and one optional one:
- Responsible Party (required): For our institution, this is always “Sponsor”.
- Sponsor (required): For our records, this will be “University of Colorado, Denver”.
- Collaborators (optional): This is where you should list any external funders, granting institutions (such as NIH institutions), and other universities or organizations working on the project with you.
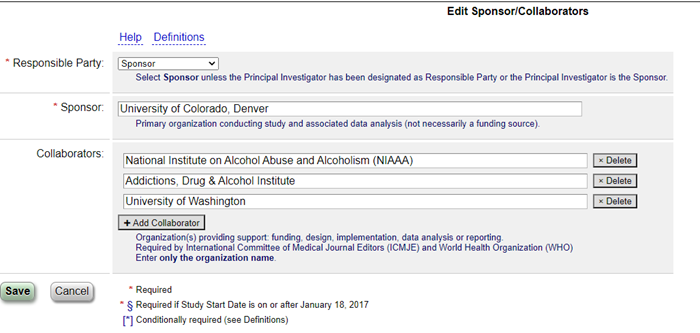
Tip! If you have an NIH grant, add your grant number as a “Secondary ID” in the Study Identification module first. This will cause the Collaborators field to show a prompt to enter the correct NIH institution name, which you can easily copy and paste into the Collaborators field.
Tags:
clinicaltrials.gov
collaborators
NIH
sponsors