Results Modules: Baseline Characteristics
Oct 21, 2021The Baseline Characteristics Module is part of the Results section of the ClinicalTrials.gov record, and includes aggregate participant demographics information, as well as study-specific baseline measurements, if applicable.
You will need to report the following information:
- Age (either with a categorical age range, or a measure of central tendency with a measure of dispersion/precision)
- Sex/Gender (count of participants)
- Race (count of participants)
- Ethnicity (count of participants)
- Region of enrollment (count of participants)
- Study-Specific Baseline Measure(s) if collected, such as participant BMI, a1C levels, baseline HAM-D scores, etc. These are reported similarly to how outcome measure data is entered in the data table, and the same QC criteria for describing the measure applies.
You will generally need to report data for participants “per arm” as well as overall data. The number of participants that you report baseline data for should match up with the numbers assigned to each arm/group in Participant Flow. If they differ, provide an explanation in the Baseline Analysis Population Description field (e.g., “baseline characteristics data was only collected for participants who received at least the first dose of the drug”).
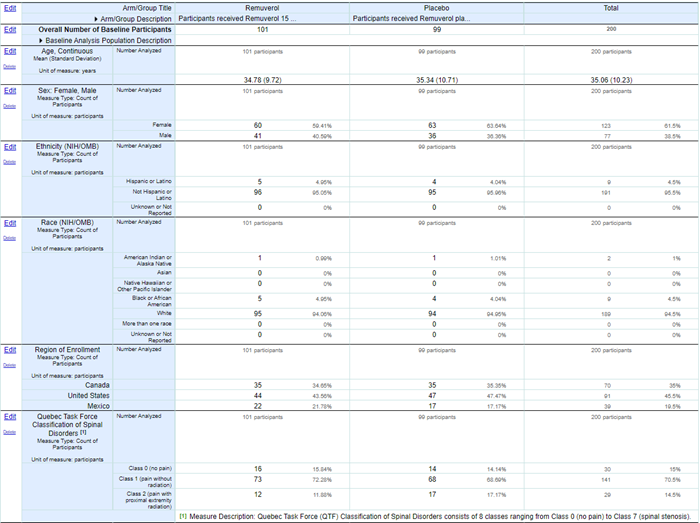
The image below shows an example study with baseline characteristics entered, including an additional Baseline Measure specific to this study: the Quebec Task Force Classification of Spinal Disorders.
Click the image to view it in a larger size.