Results Modules: Adverse Events
Jan 21, 2021When submitting results data to CliniaclTrials.gov you will need to complete 4 modules: Participant Flow; Baseline Characteristics; Outcome Measure Data, and Adverse Events.
The Adverse Events module summarizes SAEs and AEs that are collected during the study in a table. You’ll need to report:
- All-Cause Mortality: * A table of all anticipated and unanticipated deaths due to any cause.
- Serious Adverse Events: * A table of all anticipated and unanticipated serious adverse events, grouped by organ system.
- Other (Not Including Serious) Adverse Events): * A table of anticipated and unanticipated events (not included in the serious adverse event table) that exceed a frequency threshold (for example, 5 percent) within any arm of the clinical study, grouped by organ system.
Here is an example of an Adverse Events table with data entered for a crossover design study.
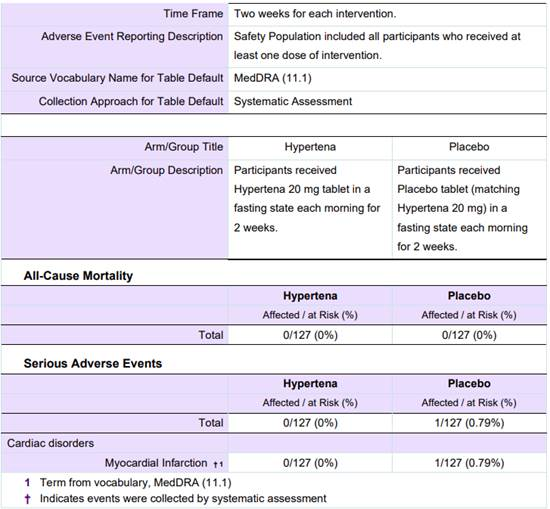
The number of participants “At Risk” for each of the three types of Adverse Events should be the total number of participants who received that intervention, or an explanation should be provided in the Adverse Event Reporting Description field.
Adverse events need to be reported PER INTERVENTION. So, if you have a crossover study with your Arms/Groups registered as:
- Arm 1: “Hypertena, then Placebo”
- Arm 2 “Placebo, then Hypertena”
For the Adverse Events module, you would need to report the Arms/Groups (as shown in the image above) as:
- Arm 1: “Hypertena”
- Arm 2: “Placebo”
This is so the data will show which intervention participants were receiving when an adverse event occurred.
Results data, if required, must be entered within 12 months of the Primary Completion Date (the last study visit where you collected data for your primary outcome measure(s)). For help entering your adverse events data or other results data, refer to the PRS Guided Tutorials for Entering Results, or contact [email protected].