My study is over. How do I close my ClinicalTrials.gov record?
Oct 22, 2020
How you close out a record for a completed or terminated study will depend on whether or not you are required to report results to ClinicalTrials.gov.
IF your study is NOT required to report results because:
- The study is not an Applicable Clinical Trial (ACT) or Probable ACT, AND
- The study is not an NIH-funded Clinical Trial (including behavioral and phase 1 trails) beginning after January 18, 2017,
Then, you may follow these steps to close the record:
Once the LAST STUDY VISIT occurs where you collected data for your primary or secondary outcome measures,
- Log in and open the record.
- Click “Open” next to the Protocol Section.
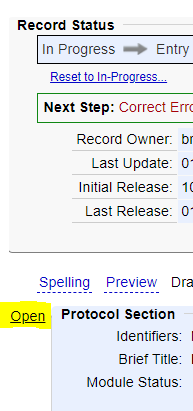
- Click “Edit” next to “Study Status”.
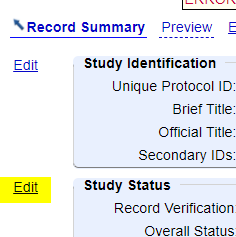
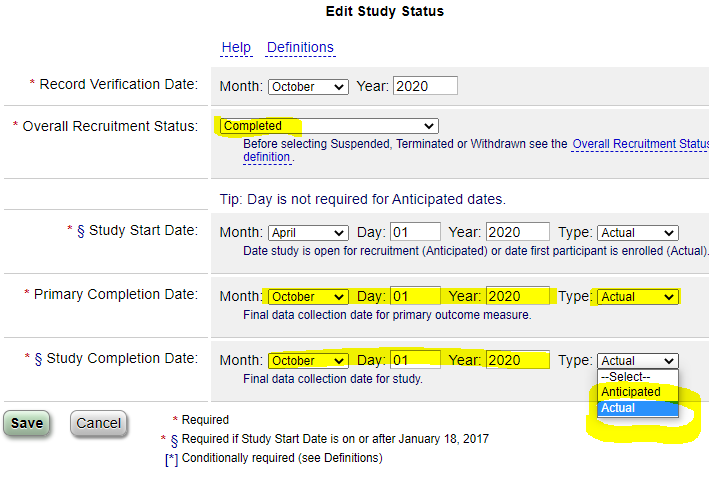
- Change the Overall Status to “Completed” (or “Terminated”, if appropriate).
- Change the “Anticipated” Primary and Study Completion dates to “Actual”, and enter the dates.
- The “Study Completion Date” is the last date when data was collected for any of the study outcomes (usually the last study visit).
- The “Primary Completion Date” is the last date when data was collected for the primary study outcome(s) (usually the same last study visit as the Study Completion Date).
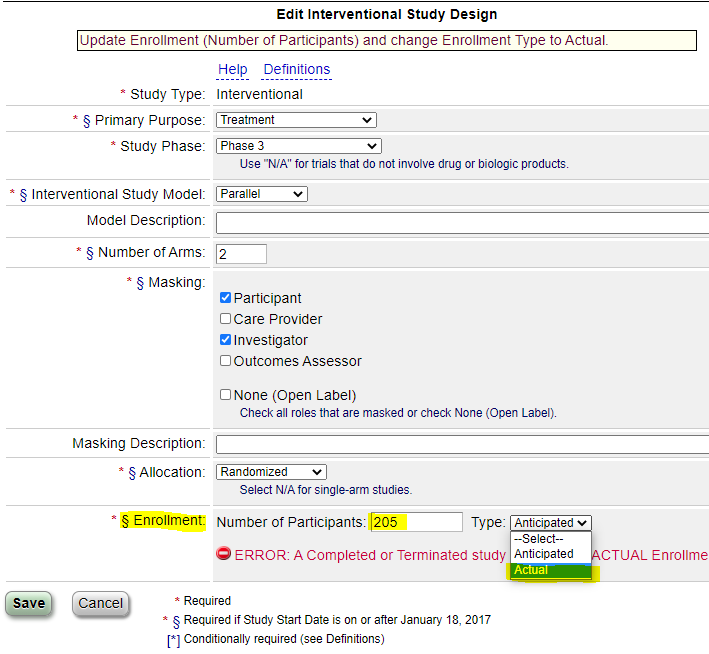
- Enter the “Actual” number of participants Enrolled under “Study Design” (the system should prompt you to do this once you complete the previous step).
- Be sure to go back to the Record Summary page and click the “Complete” button when you’re finished editing, or the record will remain in limbo.
IF your study IS REQUIRED to report results:
Complete the steps described above once the Primary Completion Date has occurred.
To close the record, you will also need to enter study results within 12 months of the Primary Completion Date. For instructions, refer to the PRS Guided Tutorials for Entering Results or contact [email protected] for help.
If required, results must be submitted whether the study terminated early or completed according to the protocol.