ClinicalTrials.gov Tip of the Week: Outcome Measures versus Protocol Outcomes
Sep 10, 2020
Outcome Measures versus Protocol Outcomes
Outcome Measures sometimes differ from how we think of outcomes in a study protocol.
In a protocol “Outcomes” might be described more like study aims, (e.g. “To determine the feasibility of enrollment in Program X” or “To determine the safety and efficacy of Drug Y”) or as clinical or behavioral measures that are not easily quantifiable, such as “MRI results” or “Survey Responses”.
For ClinicalTrials.gov purposes, Outcome Measures must be single measurable variables. This is what we mean when we say that “Outcome measures must be measurable outcomes”.
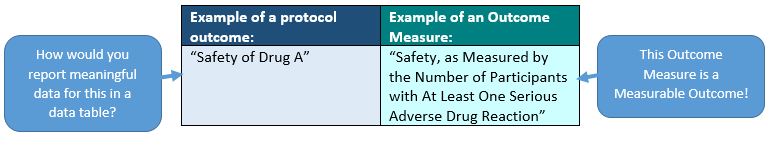
Whether you are required to report results or not, Primary and Secondary Outcome Measures must be phrased in a way that reporting them in a tabular format (where the arms/interventions are the columns and the measurements are the rows) would make sense:
.jpg?sfvrsn=7a47a4b9_2)
Think about the data you will be collecting for your study to assess your protocol outcomes and use that to inform your Outcome Measure title and description.