Audit History of your Record…Only a Click Away
Aug 26, 2021
If you are required to register your study, it is important to keep your record up to date and current.
Journal editors routinely check the audit history of the record, and changing your primary or secondary outcome measures at the end of the study can appear to be reporting bias. This can lead to difficulty in getting your results published (we’ve seen it happen!).
To view the history of the record on the public facing side, open the record, and click “Tabular View”. See below for an example:
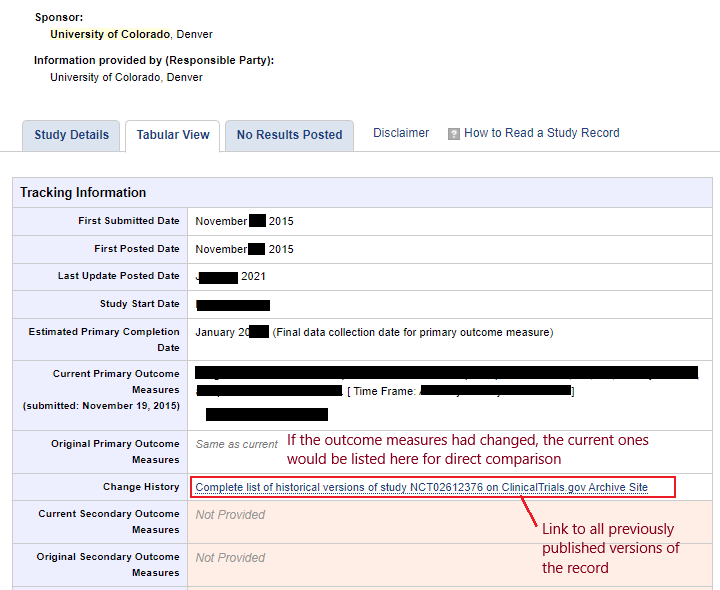
To avoid questions from journal editors, be sure to update your ClinicalTrials.gov record within 30 days of a change to any information in the record. In the case of an IRB amendment, update the record within 30 days of IRB approval of the amendment.
Journal editors routinely check the audit history of the record, and changing your primary or secondary outcome measures at the end of the study can appear to be reporting bias. This can lead to difficulty in getting your results published (we’ve seen it happen!).
To view the history of the record on the public facing side, open the record, and click “Tabular View”. See below for an example:

To avoid questions from journal editors, be sure to update your ClinicalTrials.gov record within 30 days of a change to any information in the record. In the case of an IRB amendment, update the record within 30 days of IRB approval of the amendment.
Tags:
clinicaltrials.gov
icmje
publication