Adding Study Sites & Locations to your ClinicalTrials.gov Record
Jan 27, 2022A ClinicalTrials.gov record must have at least one study location added to the record, unless the status is "Not yet recruiting" or "Withdrawn".
To add study sites and locations to your ClinicalTrials.gov record:
- Log into https://register.clinicaltrials.gov/ and open the record.
- Click "Open" next to the Protocol section
- Scroll down to the "Contacts/Locations" module and click "Edit" to open it
- Click "Add Location" and enter the study site information
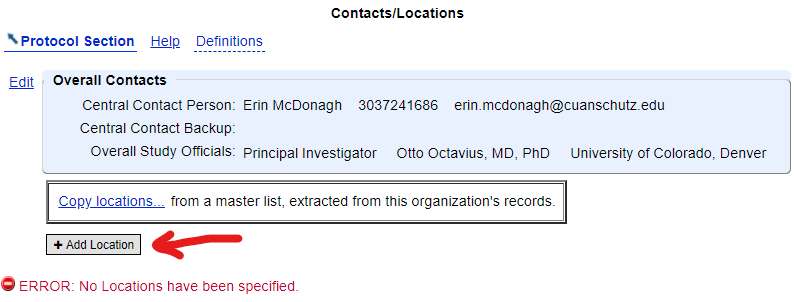
Ensuring that all study sites are added to your record is a great way to help potential participants find enrolling locations near them. All IRB-approved study sites should be added to the study record. If the overall study recruitment status is "Recruiting", at least one location must have its status set to "Recruiting" as well.
For help with your ClinicalTrials.gov records, contact [email protected].
Tags:
clinicaltrials.gov
contacts
locations
recruitment